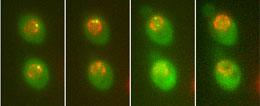
 Real-time observation of yeast genes tagged to fluoresce when transcribed into RNA could help synthetic biologists to design better circuits.Science/AAAS
Real-time observation of yeast genes tagged to fluoresce when transcribed into RNA could help synthetic biologists to design better circuits.Science/AAASJulius Lucks has heard the criticisms of synthetic biology before: life is too complicated to be manipulated by human designers; those who try have managed to cobble together only rudimentary genetic circuits from a limited suite of parts; the results are notoriously unpredictable. Meanwhile, a few high-profile successes — such as last year's creation of a bacterium with a synthetic genome1 — and enthusiastic claims that the field will solve a raft of complex health, environmental and engineering problems, only increase the pressure to deliver.
Lucks, however, is undaunted. Last month he, his wife and their young child moved across the United States, from Berkeley, California, to Ithaca, New York, where he will set up his first independent lab in the discipline at Cornell University. His optimism is representative of a new generation of synthetic biologists who are gathering to chart the course of their field this week at a conference at Stanford University in California.
Jeff Tabor, a bioengineer at Rice University in Houston, Texas, says that one goal of the conference, the fifth Synthetic Biology Meeting, is to bring more traditional molecular biologists "into the fold", both to counter their intrinsic resistance to the concept of re-engineering life and to co-opt their tools. "There is a real difference in the way that I and people younger than me see biology and think about studying cells," Tabor says, "but there are a tonne of scientists doing molecular biology work that is improving our ability to engineer biology."
For example, 'next-generation' sequencing machines, designed to vastly speed up the reading of genomes, can also offer synthetic biologists a better way to observe cellular behaviour. That in turn will help them design better circuits — for instance, by giving them a quantifiable readout of how a circuit's modifications affect its function. In a paper2 published this month by Lucks and his colleagues at the University of California, Berkeley, the group inferred the three-dimensional shapes of small RNA molecules by sequencing the corresponding DNA, using a technique called SHAPE-Seq. That strategy could help synthetic biologists to screen large pools of RNA rapidly to find those with certain structural characteristics that could be incorporated into RNA circuits.
“There needs to be a frank and open discussion about funding in synthetic biology.”
Another tool that synthetic biologists hope to adopt was published in April3 by a team led by structural biologist Robert Singer of the Albert Einstein College of Medicine in New York. By tagging particular genes with a signalling molecule that fluoresces every time the gene is transcribed, the researchers can watch and quantify transcription in real time. The work could give synthetic biologists the equivalent of an electrician's circuit tester, helping them to engineer more predictable biological circuits.
"Using a technology like this, you can see exactly what a circuit is doing and count the number of circuit signals that are being produced in real time in live cells," Tabor says. "This is exactly what we need to help us put circuits together."
Bioengineer Adam Arkin, Lucks' mentor at Berkeley, has pursued the idea that circuits can be made more reliable by basing parts on existing cellular components that already accomplish a certain function in the cell. Such 'mother parts' could be tweaked slightly to yield 'families' of parts with similar features that could carry out their functions independently and efficiently.
In April, the team published a proof of concept for this approach4 in which they tweaked an RNA-based gene-regulation system to simultaneously control the expression of multiple genes in a cell from the bacterium Escherichia coli, and even make a simple RNA circuit. Because the system is entirely RNA-based, it eliminates the need to translate a messenger RNA into a protein regulator, thereby reducing the overall complexity of the system.
Another approach to complexity involves designing multicellular circuits in which each cell is a circuit component. This neatly skirts the dilemma of trying to insulate the parts of a circuit from one another within the cytoplasm of a single cell. Chris Voigt, who is moving from the University of California, San Francisco, to co-direct a new synthetic-biology institute at the Massachusetts Institute of Technology in Cambridge, has been pursuing this approach in his lab with colleagues who published their proof of principle last December5. "There's been a change in the scale of the problems that we can address, and this comes out of the tools that synthetic biology can provide," says Voigt. At the meeting, Voigt will describe his lab's attempts to re-engineer the way some organisms convert nitrogen into a useable form through a molecular pathway that involves dozens of genes.
Synthetic biologists have been organizing their own initiatives to tackle other obstacles. For instance, one of the field's key tenets is that off-the-shelf molecular 'parts' could be used to program cells to carry out specific functions, such as making a drug or a biofuel. But such ambitious goals depend on the quality of the available parts6. So, in late 2009, an initiative called the BIOFAB (International Open Facility Advancing Biotechnology), funded by the US National Science Foundation, began working to design reliable parts with known functions. The BIOFAB has now made about 3,000 well- characterized parts and has released around 500 as a higher-quality curated collection.
ADVERTISEMENT
Yet money is scarce for this kind of work — a challenge to be addressed at a conference workshop that will include funding agencies and industry. "There needs to be a frank and open discussion about funding in synthetic biology, especially in the United States," says Pam Silver, a systems biologist at Harvard University in Boston, Massachusetts. The bread-and-butter work that the field needs, such as fine-tuning circuitry, is more applied than most 'hypothesis-driven' research that is the remit of agencies such as the US National Institutes of Health. And most funders want applicants to focus on specific agendas, such as health or biofuels.
Indeed, Rob Carlson, a principal at the engineering, consulting and design company Biodesic in Seattle, Washington, wonders whether the field of synthetic biology is big enough to become a well-oiled engineering machine. This week's conference is sold out at 700 attendees, with a waiting list of at least 100, but as Carlson points out, many of those attending will be reporters and investors.
"Given the complexity of the task at hand, it doesn't surprise me at all that we are still going slowly," says Carlson.
-
References
- Gibson, D. G. et al. Science 329, 52-56 (2010). | Article | PubMed | ISI | ChemPort |
- Lucks, J. B. et al. Proc. Natl Acad. Sci. USAdoi:10.1073/pnas.1106501108 (2011).
- Larson, D. R., Zenklusen, D., Wu, B., Chao, J. A. & Singer, R. H.Science 332, 475-478 (2011). | Article | PubMed | ISI | ChemPort |
- Lucks, J. B., Qi, L., Mutalik, V. K., Wang, D. & Arkin, A. P. Proc. Natl Acad. Sci. USA 108, 8617-8622 (2011). | Article | PubMed |
- Regot, S. et al. Nature 469, 207-221 (2011). | Article | PubMed | ISI | ChemPort |
- Kwok, R. Nature 463, 288-290 (2010). | Article | PubMed | ISI | ChemPort |